Abstract
Background and Aims— We performed a phase 2 trial to determine whether mechanical thrombectomy (MT) alone in acute ischemic stroke patients demonstrates “promise” or a lack thereof (“futility”) compared with those treated with intravenous (IV) recombinant tissue plasminogen activator (rt-PA) (historical controls).
Methods— Subjects with a baseline National Institutes of Health Stroke Scale (NIHSS) score ≥6 who presented within 4.5 hours of symptom onset with documented occlusion of intracranial internal carotid artery, or M1 or M2 segment of the middle cerebral artery on computed tomographic angiography were treated with MT alone. The primary outcome was functional independence defined by a modified Rankin scale (mRS) of 0-2 at 90 days post recruitment.
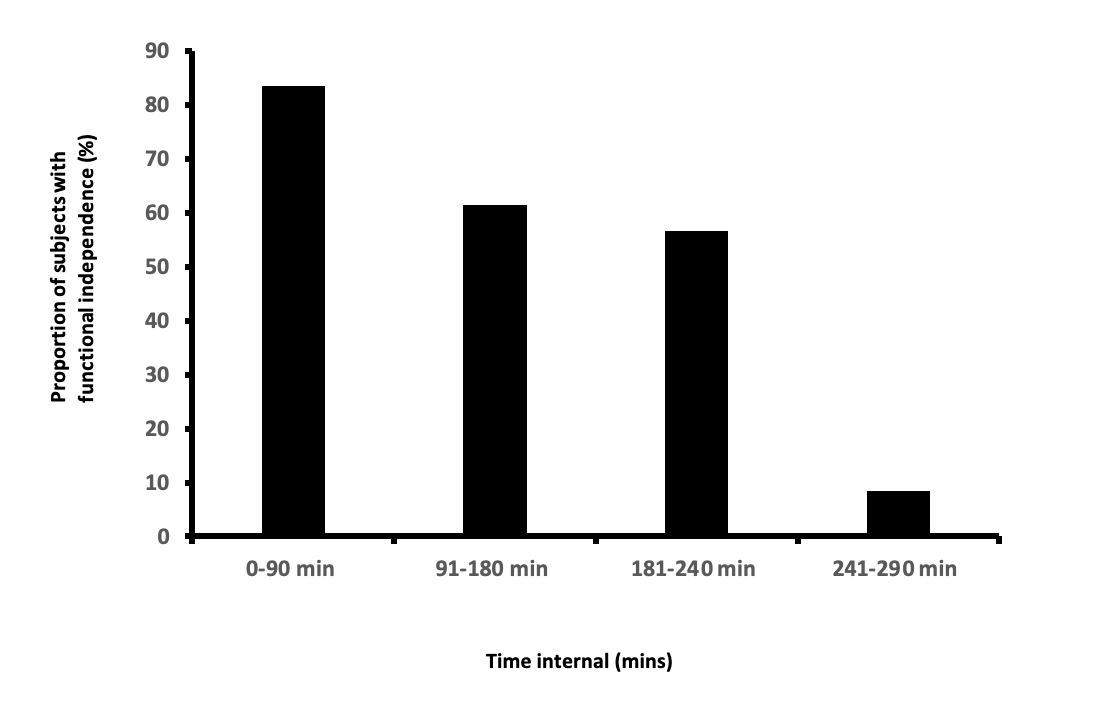
Results— The 72 subjects (median baseline NIHSS score of 17) were treated with MT with a median time from symptom onset of 180 minutes with complete and partial angiographic recanalization observed in 45 and 27 patients, respectively. Symptomatic intracerebral hemorrhage (≥4 points NIHSS score increase) within 24 hours was observed in none of the 72 subjects. Overall, functional independence at 90 days was observed in 38 (52.8%) of 72 subjects. The rate was higher than the rate of functional independence in a comparable historical cohort of patients treated with IV rt-PA (52.8% versus 35.9%, p=0.032) enabling us to reject the futility hypothesis.
Conclusions— Due to the relatively high rates of functional independence at 90 days observed in this phase 2 trial, a randomized phase 3 comparing standard intravenous rt-PA followed by MT with primary MT is being planned.

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Copyright (c) 2022 Journal of Vascular and Interventional Neurology